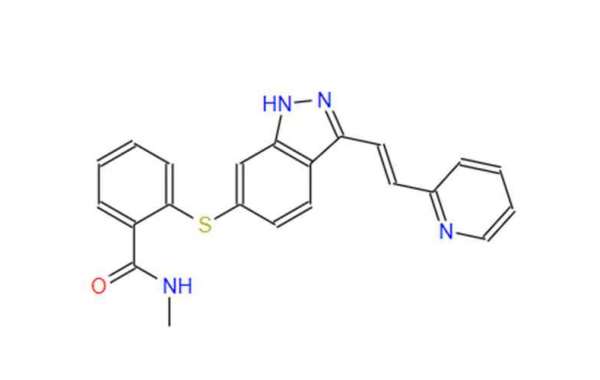
This blog post delves into the complexities of Axitinib API production, specifically focusing on the challenges associated with quality control (QC) and quality assurance (QA).
The Importance of Quality in Axitinib API Manufacturing
Axitinib, a highly potent tyrosine kinase inhibitor, demands stringent quality control measures to guarantee patient safety and drug efficacy. Even minor variations in the API's purity, structure, or consistency can significantly impact the drug's effectiveness and potentially lead to adverse side effects.
QC and QA in Axitinib API Production: Hurdles to Overcome
Several key challenges confront manufacturers striving to uphold the highest quality standards in Axitinib API production:
Synthetic Complexity: Axitinib's intricate chemical structure necessitates a multi-step synthesis. Each step introduces potential sources of variability that must be meticulously monitored and controlled.
Impurity Management: The presence of even trace amounts of impurities can significantly impact Axitinib's efficacy and safety. QC techniques like high-performance liquid chromatography (HPLC) and mass spectrometry (MS) are crucial for detecting and quantifying impurities.
Polymorphism: Axitinib exists in various solid forms (polymorphs) with potentially different properties. Ensuring consistent production of the desired polymorph is essential for maintaining a predictable drug release profile.
Degradation Control: Axitinib is susceptible to degradation under certain conditions like light or heat. Implementing robust QA measures, such as proper storage and handling protocols, is vital to minimize degradation throughout the manufacturing process.
Strategies for Ensuring Axitinib API Quality
Combating these challenges requires a comprehensive approach that integrates robust QC practices with a rigorous QA framework:
Process Validation: Thorough validation of each step in the synthesis ensures reproducibility and minimizes the risk of inconsistencies.
In-Process Controls: Implementing real-time monitoring systems throughout the manufacturing process allows for immediate identification and rectification of any deviations from quality standards.
Statistical Process Control (SPC): Utilizing SPC techniques helps manufacturers identify trends and potential issues before they escalate into major quality control problems.
Continuous Improvement: A culture of continuous improvement that encourages ongoing research and development of novel QC and QA methodologies is essential for maintaining the highest quality standards.
The Road Ahead: Ensuring Patient Safety Through Quality
The challenges associated with Axitinib API production necessitate constant vigilance and innovation. By implementing robust QC and QA strategies, manufacturers can ensure the consistent delivery of a safe and efficacious Axitinib API, paving the way for patients to access this life-changing medication with utmost confidence.
Disclaimer: This blog post is for informational purposes only and should not be taken as medical advice. Please consult with a healthcare professional for any questions or concerns regarding Axitinib or your individual health needs.